Research Projects
Intervention Strategies to Treat Placental Dysfunction
Despite our best efforts, research has so far failed to provide efficient, personalized solutions to both predict and treat placental dysfunction. We believe our lack of understanding of disease mechanisms is the cause of this problem.
Current treatments focus on the management of symptoms to prolong pregnancy and improve outcome. Treating pregnant patients always requires appropriate caution to ensure safety for both the fetus and the mother. The risk of potentially doing more harm is high, making appropriate clinical trials that assess both short and long term risks difficult. Not surprisingly, there is little research pursuing new therapeutic tools and medications that could improve pregnancy outcomes.
To overcome these challenges our work aims to provide in vitro evidence about the potential effectiveness of existing medicines to inform patients and inspire clinician scientists of the potential opportunities available.
Heparin and placental function
Heparin is a drug often given to improve blood flow, reduce placental infarction and prevent recurrent pregnancy loss. The exact mechanism of how heparin acts on the placenta has proven elusive since little research has been done in this particular area. This gap is complicated by the fact that heparin can bind and influence the actions of many molecules in the human body beyond its actions on coagulation. A study by Rey et al. suggested that women who would not suffer from thrombophilia (increased clotting of the blood) would benefit from treatments with heparin during pregnancies. This work and other sparked our interest in the non coagulation dependent aspects of heparin for pregnancy where we try to understand the actions on the placenta and the physiology of the mother.
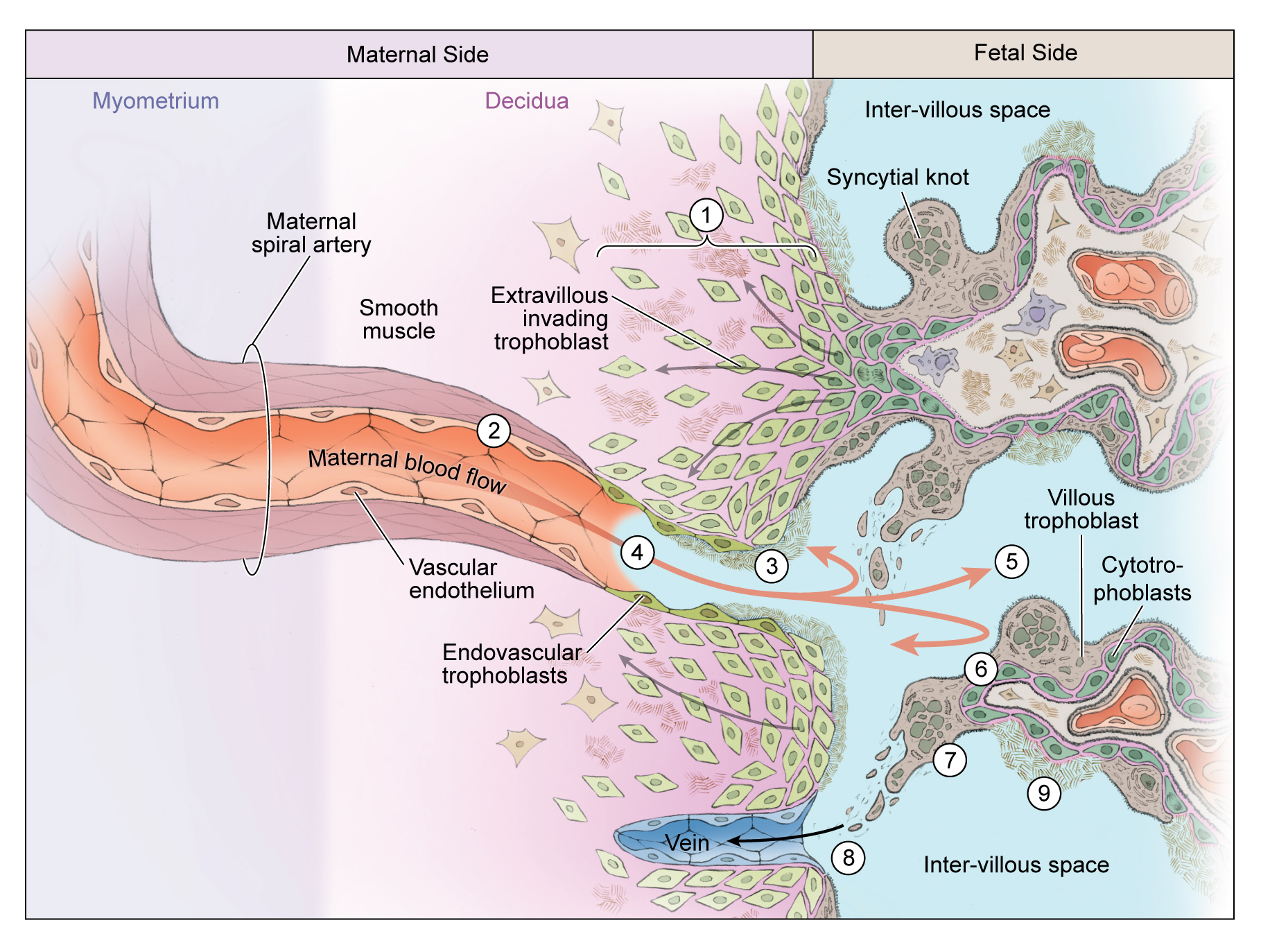
Normal placental development. Extravillous cytotrophoblasts proliferate in anchoring columns to successful invade through the decidua (1) and transform the distal spiral arteries (2). These changes mediate high volume flow at low pressure into the intervillous space (3). The placental villi are covered by the villous trophoblast compartment (4), comprising cytotrophoblasts that proliferate to generate the outer syncytiotrophoblast in direct contact with maternal blood.
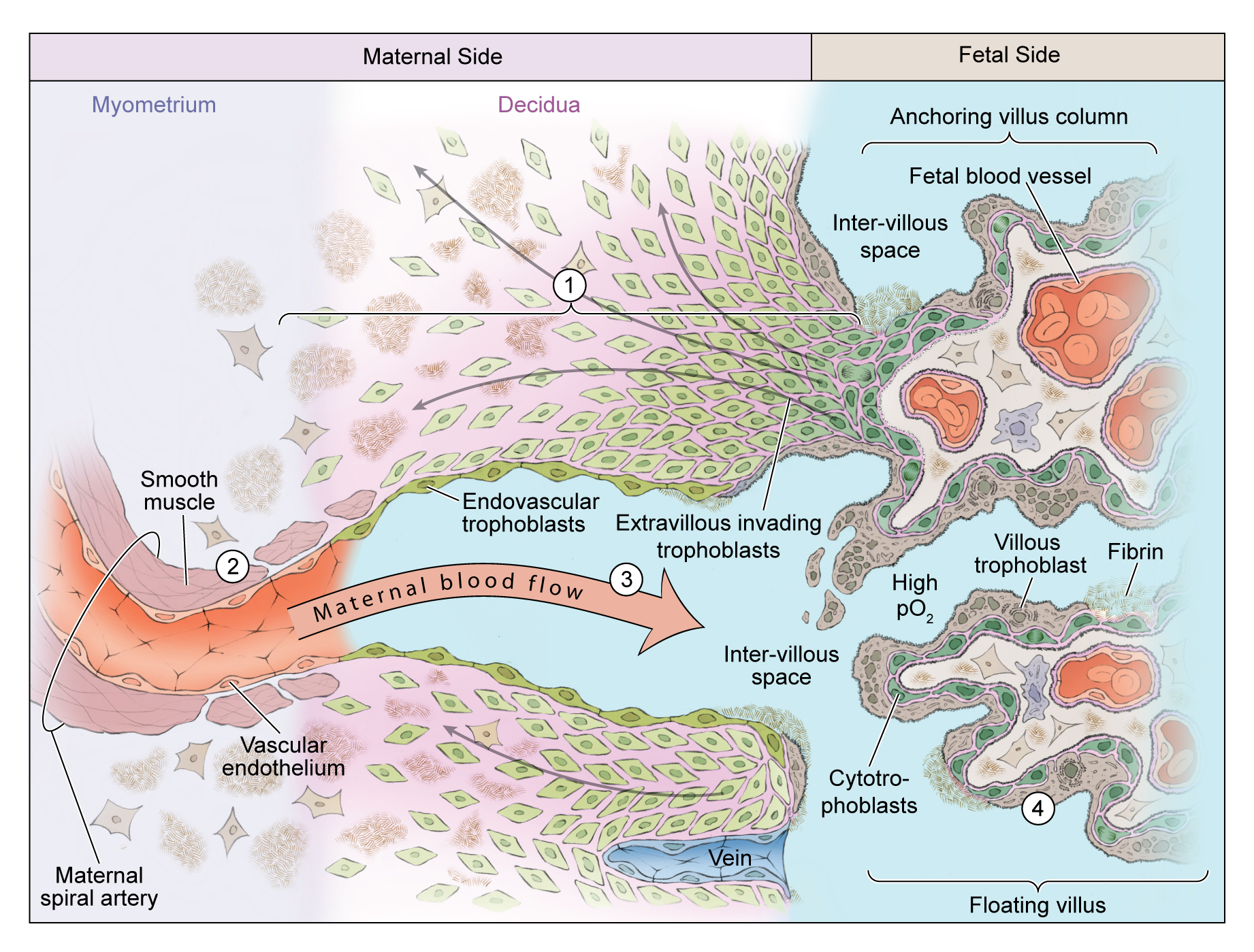
Uteroplacental vascular insufficiency. Extravillous cytotrophoblasts are less successful in invading the maternal decidua and may be removed by the maternal immune system (1). Consequently the distal spiral arteries are narrower (2) and diseased, accompanied by atherosis or local fibrin deposition (3) and reduced endovascular invasion (4). Hypoxia or hypoxia-reoxygenation injury (5) has direct effects on the villous trophoblast compartment, reducing syncytial fusion (6) that may trigger the formation of syncytial knots (7). These accumulate but may fragment and shed into maternal blood (8), whereas areas deficient in syncytial fusion may exhibit focal necrosis (9).
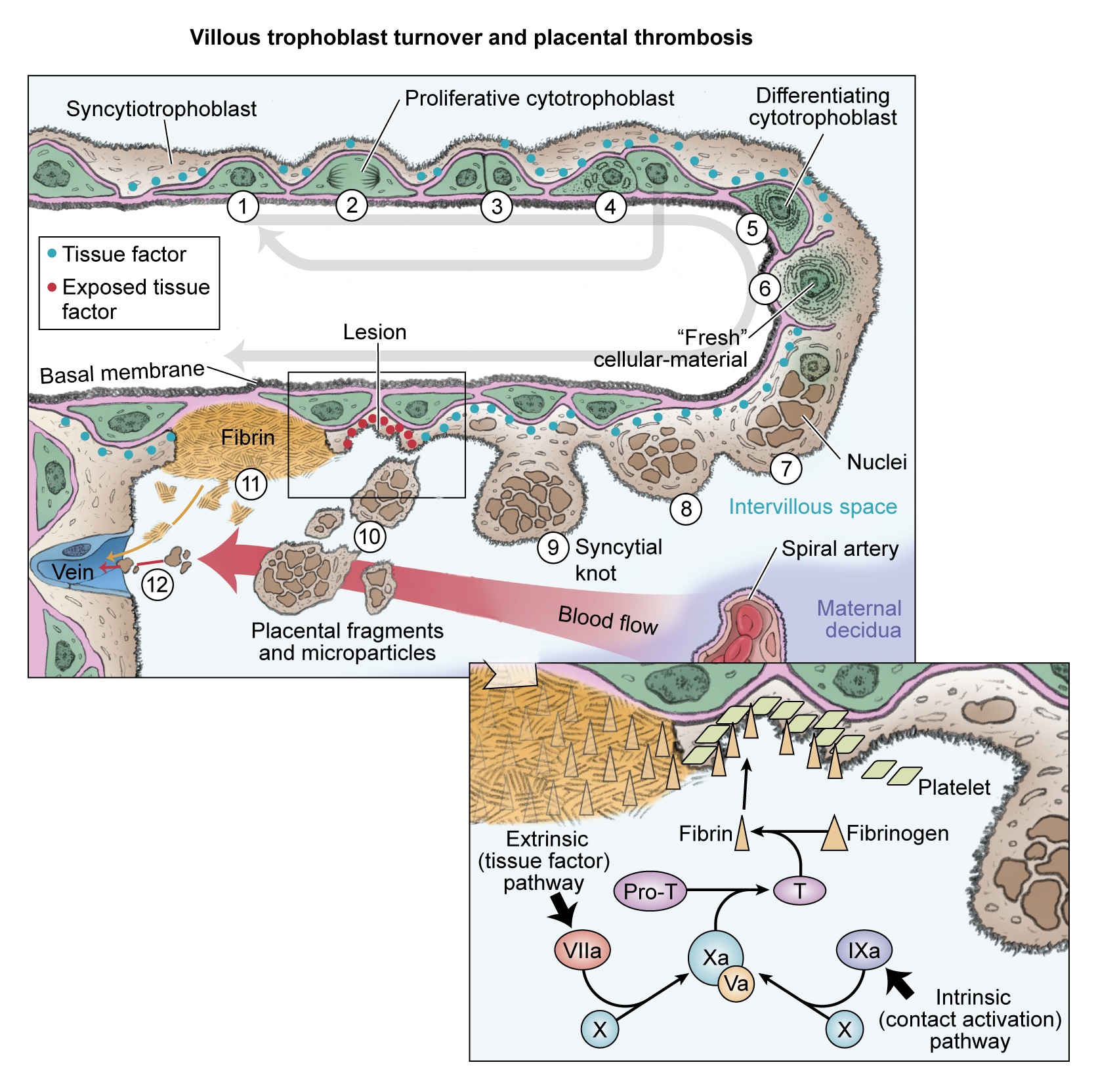
Abnormal villus trophoblast differentiation and placental thrombosis. Progenitor cytotrophoblasts proliferate (1) and divide asymmetrically (2 and 3) so that the progenitor is conserved (4) for subsequent rounds of syncytial fusion. The daughter cytotrophoblast prepares for syncytial fusion (5), focally donating transcriptional machinery and antiapoptotic proteins into the outer syncytiotrophoblast (6). Syncytial nuclei gradually progress toward apoptosis and aggregate in syncytial knots (7 and 8). Syncytial fusion is restricted in severe PE, increasing the number and size of syncytial knots (9 and 10). Some knots may fragment into the intervillous space, whereas other parts of the abnormal villi have exposed cytotrophoblasts and basal lamina (11) that trigger local thrombosis (see inset). Syncytiotrophoblast fragments are filtered in the maternal lungs, whereas microparticles pass through and may exert systemic effects in the maternal vasculature.
For full text and references please refer to Kingdom and Drewlo, 2011 Blood Journal
Rosiglitazone - a novel target in abnormal pregnancy?
The anti-diabetic drug Rosiglitazone recently has drawn some attention and a renaissance. First, it was re-introduced to the market after it was shown that there were no long term cardiovascular effects. Second, researchers, including our group, have recently investigated its potential effect on human reproduction, in general and placentation, specifically.
The actions of Rosiglitazone are well known for the treatment of diabetes. We are currently investigating the possibility that this drug class might be able to improve placental function in certain placenta insufficiency syndromes. This in-vitro study was recently funded by the National Institute of Health (R01HL128628-01 to Dr. Drewlo) and the prestigious March of Dimes Basil O'Connor Scholar Award.
We are currently assembling some exciting data on this topic. Make sure to come back soon and look out for #DrewloPub online via twitter.